Breaking News! China’s First Domestically Developed IABP Approved for Market Launch
2025-01-13
On January 13, Anhui Tongling BionicsTechnology Ltd. officially obtained the Class III Medical Device Registration Certificate (National Registration No. 20253080105) for its innovative TL-IABP-100 Intra-Aortic Balloon Pump, marking its approval for market release. This breakthrough makes it China’s first fully independent intellectual property-protected IABP, poised to enhance the accessibility and application of IABP technology, benefiting more patients.

IABP: A Lifesaving "Guardian" for Critically ill Patients
The Intra-Aortic Balloon Pump (IABP) provides cardiac support for patients with severe cardiovascular conditions. Its working principle is straightforward: A balloon-tipped catheter is placed in the descending aorta, and an external console inflates and deflates the balloon in sync with the cardiac cycle (diastole and systole). This augments cardiac output, improves coronary perfusion, and stabilizes hemodynamics.
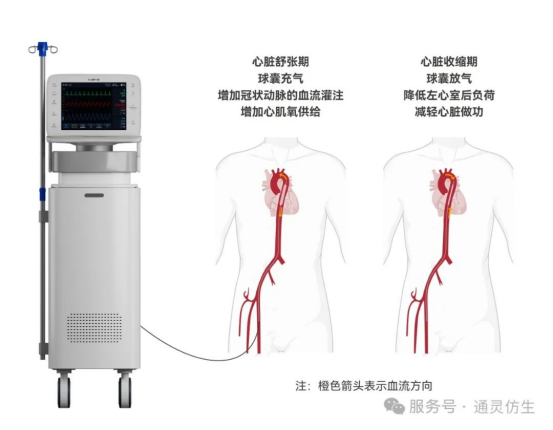
Extensive clinical studies worldwide confirm that IABP is a safe and effective cardiac assist device for indicated patients, significantly improving survival rates. It remains the most widely used and clinically validated mechanical circulatory support system to date.
Advanced Features for Diverse Clinical Needs
Tongling Bionics, leveraging cutting-edge technology and years of R&D, has successfully developed the TL-IABP-100. This system offers two operation modes (navigation and manual) and employs big data-driven ECG analysis to enable multiple triggering modes, ensuring timely and precise counterpulsation therapy. Beyond core functions, it integrates differentiated features to meet varied clinical demands, including:
1.User management: Add, search, modify, or export patient data; review historical treatment records.
2.Data export: Export therapy logs and system data for post-analysis.
3.Dual operation: Tactile physical buttons + touchscreen.
4.Long-lasting battery: High-capacity rechargeable lithium battery for extended support.
5.One-click weaning: Automated weaning protocol.
6.System self-test: Ensures readiness upon startup.
7.Adjustable assist ratios: Smooth transitions with superior adaptability.
……
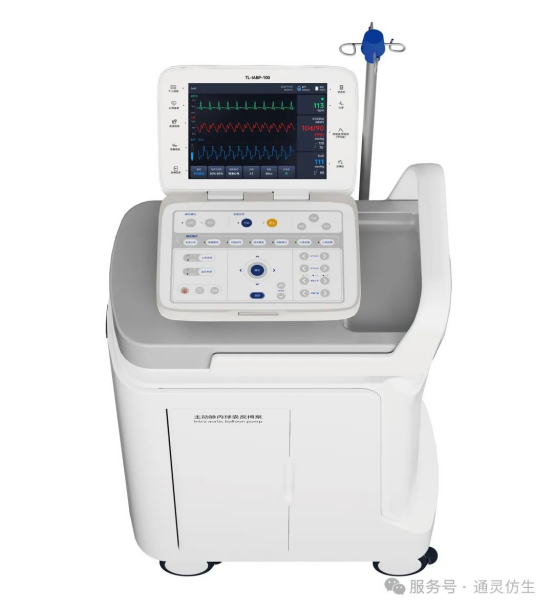
The TL-IABP-100 features an HD touchscreen for real-time visualization of waveforms, ECG, and blood pressure. Its ergonomic button layout ensures intuitive operation, while a built-in printer generates reports on waveforms, signal sources, and trigger modes (with optional timed auto-printing). The system also includes three-tier alarms and a comprehensive help database for rapid troubleshooting.
Tongling Bionics: Integrated Heart Failure Diagnosis, Treatment & Rehabilitation
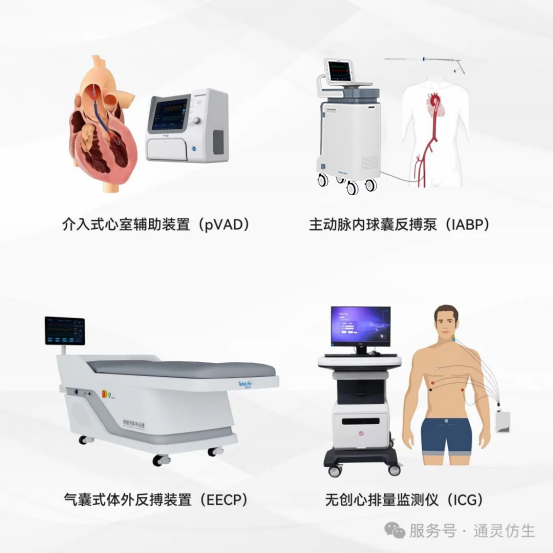
Committed to becoming a global leader in holistic heart failure solutions, Tongling Bionics has expanded its portfolio beyond IABP, including:
TL-EECP-100: A enhanced external counterpulsation device for cardiovascular rehabilitation.
TL-NiCOM-100: A non-invasive cardiac output monitor for real-time heart function assessment.
pVAD (under clinical trials): A percutaneous ventricular assist device for advanced circulatory support.
Looking ahead, the company will uphold its mission—"Harnessing Technology for the Grand Beauty of Life"—by accelerating product deployment and upgrades, strengthening its heart failure product line, maintaining its pioneering edge, and driving innovation to safeguard human health.
Latest News



